Next: Covalent angles Up: Description of the molecular Previous: Molecular geometry and non-bonded Contents
Two types of bonds are implemented, standard harmonic bond (Note: no factor 1/2 in the potential):
and Morse potential:
Harmonic bonds may be also used for description of the so-called Urey-Bradley term which is a harmonic potential between the 1-3 neighbors (atoms separated by two covalent bonds). This term is included for example into CHARMM force field as a part of interaction potential for covalent angles.
The first line of the bond section of a .mmol file is the number
of bonds. Then lines, one per each bond, follow with parameters for each bond.
Each line consists of the following fields:
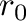 in Å. It may be
set negative, in such a case the equilibrium bond length will be taken
from the corresponding coordinates in the fisrt section of the
in Å. It may be
set negative, in such a case the equilibrium bond length will be taken
from the corresponding coordinates in the fisrt section of the
.mmol file.
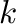 in
in 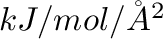
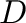 (
(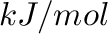 ) and
) and 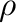 (
(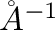 ) parameters
of the Morse potential respectively
) parameters
of the Morse potential respectively